The ROADSTER Study
Prospective, single arm, multi-center trial of the TCAR Procedure using the ENROUTE® Transcarotid NeuroprotectionSystem (NPS) in high surgical risk patients with carotid artery stenosis.
“The overall stroke rate of 1.4% is the lowest reported to date for any propsective multi-center trial of carotid stenting.”
– J Vasc Surg 2015;62:1227-35
Results of the ROADSTER study
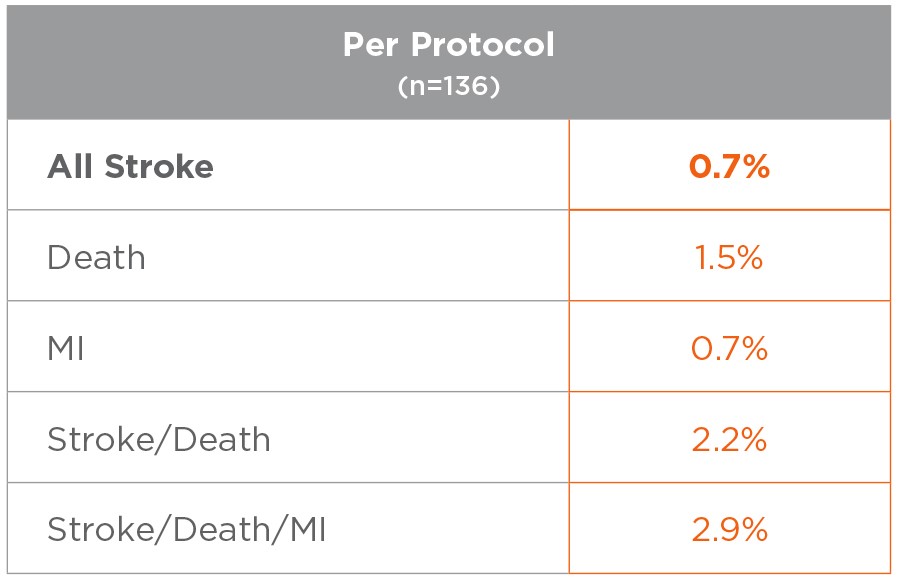
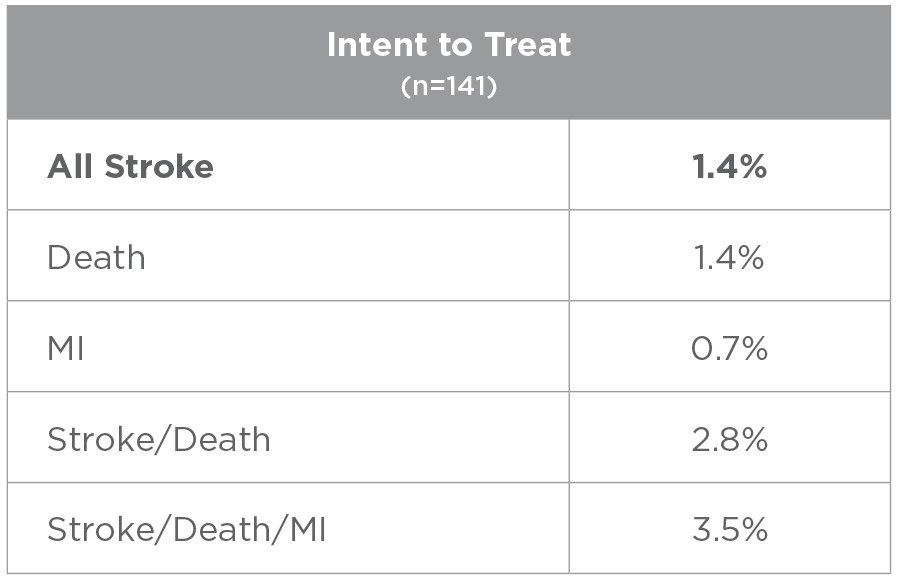
Cranial Nerve Injury Rates

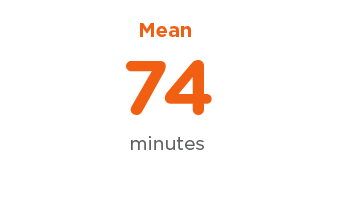
Transcarotid Efficiency

Procedure Time
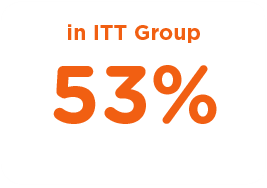
Local Anesthesia Use
Related publications

Results of the ROADSTER Multicenter Trial of Transcarotid Stenting with Dynamic Flow Reversal.
Kwolek CJ et al.

One-Year Results of the ROADSTER Multicenter Trial of Transcarotid Stenting with Dynamic Flow Reversal
Mahmoud Malas et al.

Pre- and Post-TCAR Characteristics of Common Carotid Artery: A Post Hoc Analysis of ROADSTER-1
Raghu L. Motaganahalli et al.

The ROADSTER Investigational Device Exemption Trial Leads to FDA Approval of the First Stent Labeled for TCAR
Manish Mehta et al.
Discover more clinical studies

First in man results using ENROUTE® Transcarotid Neuroprotection System.

Post Approval registry validating safety and efficacy of the ENROUTE Stent.

Real world population data validating CEA-like outcomes.
Validation of superior outcomes
to TF-CAS.

