The ROADSTER 2 Clinical Study
This study evaluated usage of the ENROUTE® Transcarotid Stent System in conjunction with the ENROUTE® Transcarotid Neuroprotection System in high surgical risk patients with carotid artery stenosis. 692 patients were treated across 43 sites, with 81% of the physician enrollers being new TCAR operators.
“The stroke rate of 0.6% after TCAR in the Per Protocol population may be the lowest reported rate after any carotid intervention.”
–Stroke 2020; 51:2620–2629
“The stroke rate of 0.6% after TCAR in the Per Protocol population may be the lowest reported rate after any carotid intervention.”
–Stroke 2020; 51:2620–2629

Results of the ROADSTER 2 Study
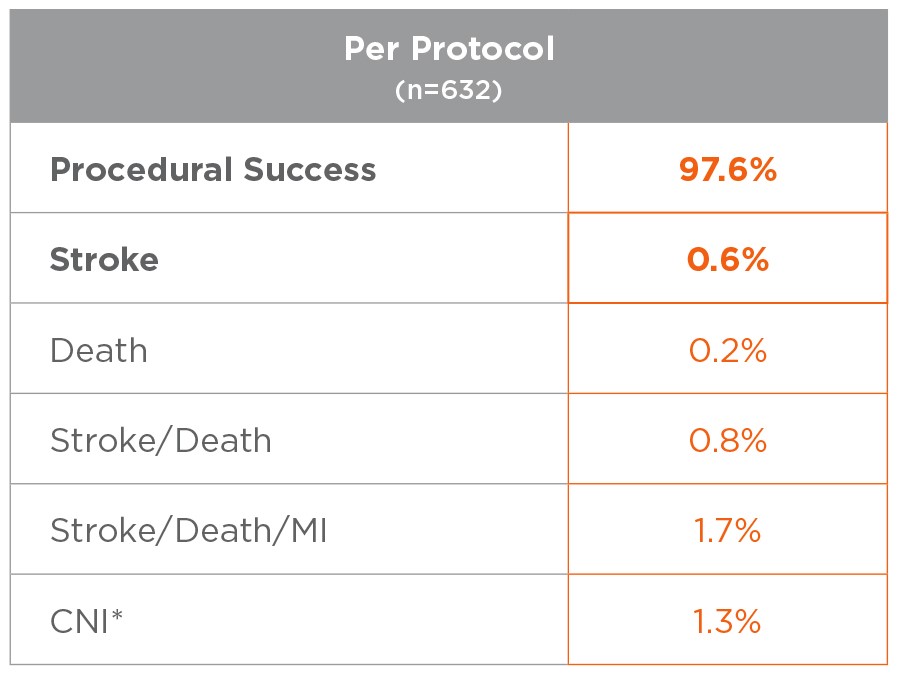
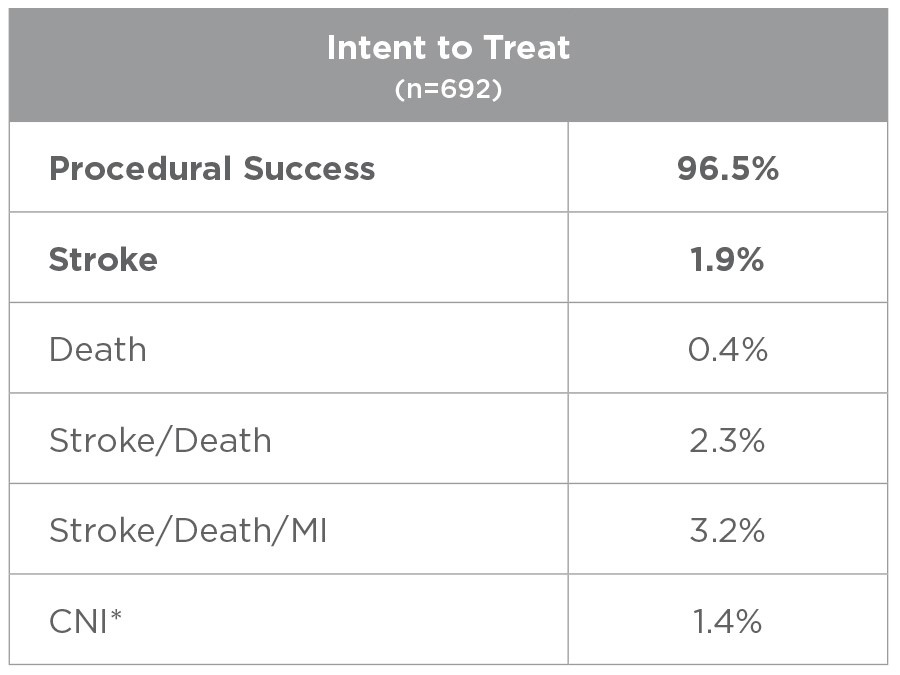
*All CNI injuries resolved in patients that consented to an extended follow up period.
0.6% stroke rate in the FDA Analysis Population (PP)
81% of Physicians were new to TCAR
Excellent outcomes achievable with adherance to Protocol

TCAR continues to show a low stroke and death rate with lower rates of cranial nerve injury compared to CEA, which we believe will further instill physician confidence and adoption.”
Vikram Kashyap, MD – University Hospitals Case Medical Center, Cleveland, OH, Chief of Vascular Surgery and Endovascular Therapy
Related publications

Results of the ROADSTER Multicenter Trial of Transcarotid Stenting with Dynamic Flow Reversal
Kwolek CJ et al.

One-Year Results of the ROADSTER Multicenter Trial of Transcarotid Stenting With Dynamic Flow Reversal
Mahmoud Malas et al.

Pre- and Post-TCAR Characteristics of Common Carotid Artery: A Post Hoc Analysis of ROADSTER-1 Trial
Raghu L. Motaganahalli et al

The ROADSTER Investigational Device Exemption Trial Leads to FDA Approval of the First Stent Labeled for TCAR
Manish Mehta et al.
Discover more clinical studies

First in man results using ENROUTE® Transcarotid Neuroprotection System.

Pre-Market Approval of the ENROUTE Neuroprotection System.

Real world population data validating CEA-like outcomes.
Validation of superior outcomes
to TF-CAS.

