The ROADSTER 3 Clinical Study
First-ever prospective, multicenter, trial evaluating the safety and effectiveness of TCAR using the ENROUTE® Transcarotid Stent System (TSS) in conjunction with the ENROUTE® Transcarotid Neuroprotection System (NPS) for the treatment of carotid stenosis in standard surgical risk patients.
This Data is the Latest in a
Line of ROADSTER Trials
Demonstrating Consistent,
Low Adverse Event Rates
Across All Risk Levels.
This Data is the Latest in a Line of ROADSTER Trials Demonstrating Consistent, Low Adverse Event Rates Across All Risk Levels.

Results of the ROADSTER 3 Study
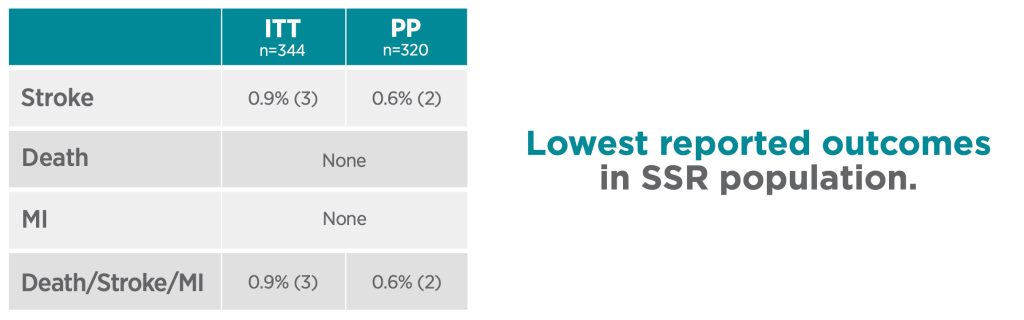
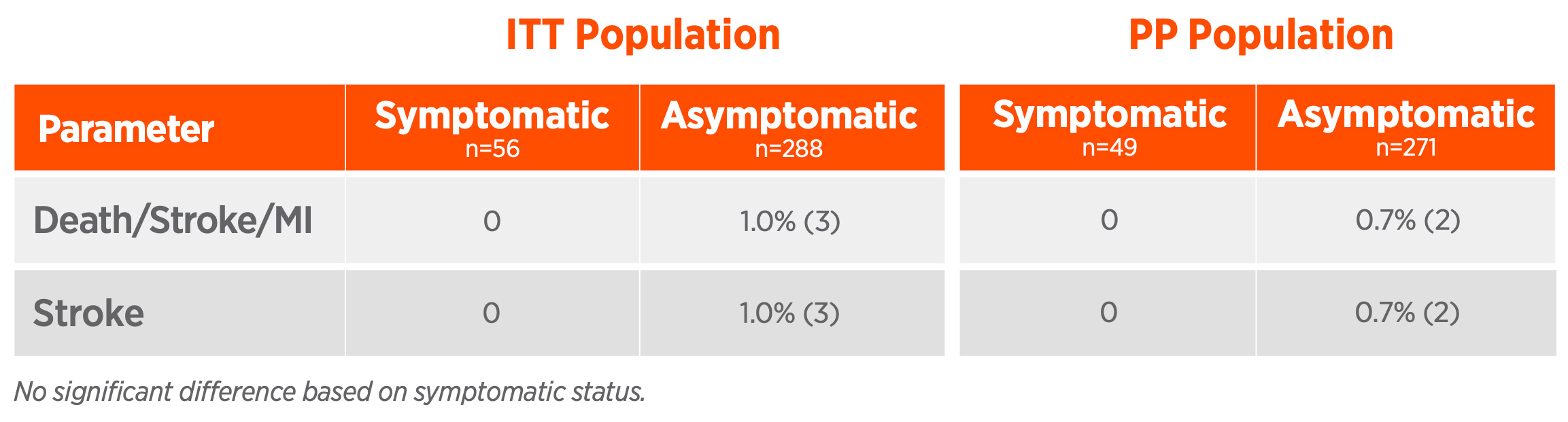
30-day results of the ROADSTER 3 study demonstrate that TCAR, using the ENROUTE TSS in conjunction with the ENROUTE NPS, is safe and effective in patients at standard risk for adverse events from carotid endarterectomy. With indisputable clinical and patient benefits, TCAR is the less invasive standard in stroke prevention in patients requiring carotid artery disease intervention.

Related publications

Results of the ROADSTER Multicenter Trial of Transcarotid Stenting with Dynamic Flow Reversal
Kwolek CJ et al.

One-Year Results of the ROADSTER Multicenter Trial of Transcarotid Stenting With Dynamic Flow Reversal
Mahmoud Malas et al.

Pre- and Post-TCAR Characteristics of Common Carotid Artery: A Post Hoc Analysis of ROADSTER-1 Trial
Raghu L. Motaganahalli et al

The ROADSTER Investigational Device Exemption Trial Leads to FDA Approval of the First Stent Labeled for TCAR
Manish Mehta et al.
Discover more clinical studies

First in man results using ENROUTE® Transcarotid Neuroprotection System.

Pre-Market Approval of the ENROUTE Neuroprotection System.

Real world population data validating CEA-like outcomes.
Validation of superior outcomes
to TF-CAS.

