ENROUTE® Transcarotid
Stent System
Stroke Prevention through Plaque Stabilization
First and only carotid stent system designed and indicated for transcarotid access
Designed with purpose
Designed for TCAR®, the ENROUTE Transcarotid Stent System autoconforms to anatomy and delivers long-term plaque stabilization to prevent future strokes
Features include:
- TCAR specific delivery length: Short 57cm delivery system for ergonomic and precise stent delivery
- Optimized cell design: Provides the most coverage of any open cell stent design, autoconforming to even the most difficult anatomies
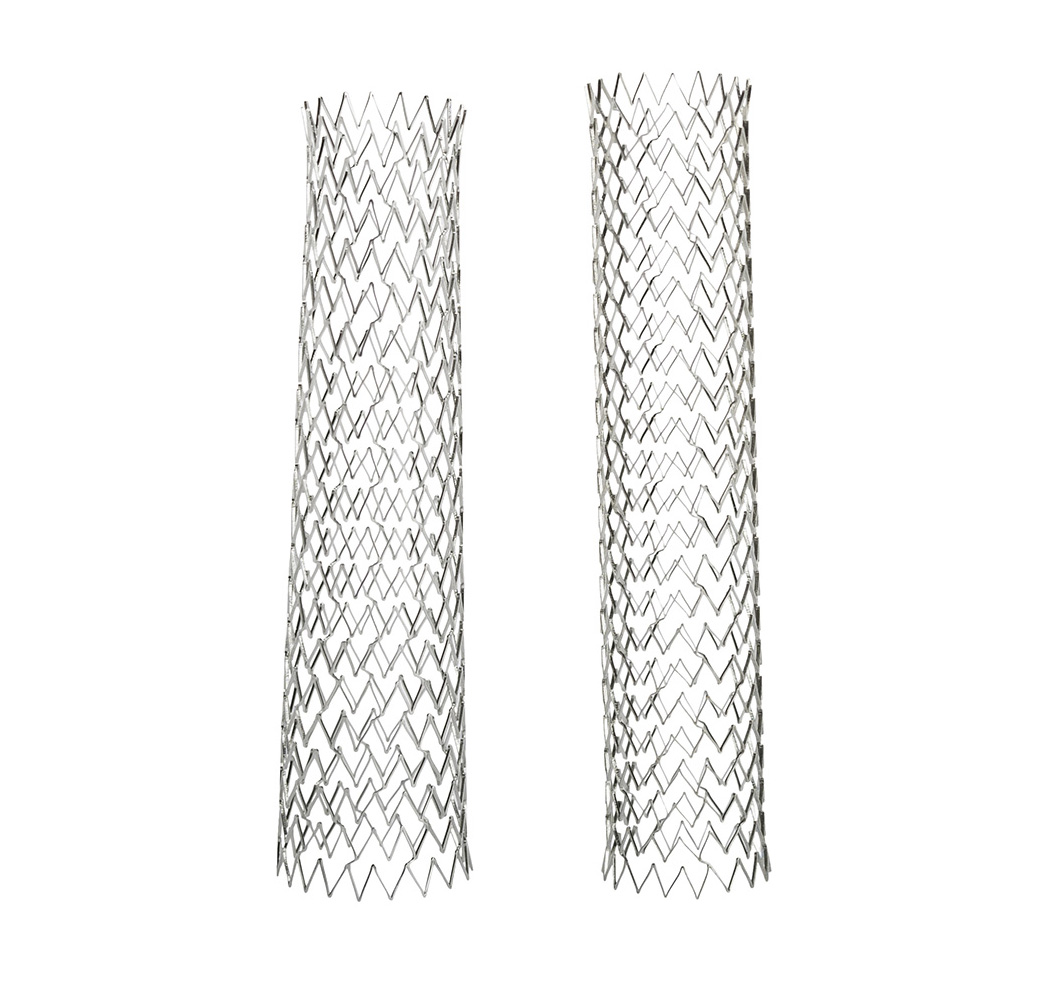
See its effectiveness

Conformable
Optimized hybrid-cell stent design autoconforms in 2mm segments for uniform scaffolding that respects native vessel anatomy.
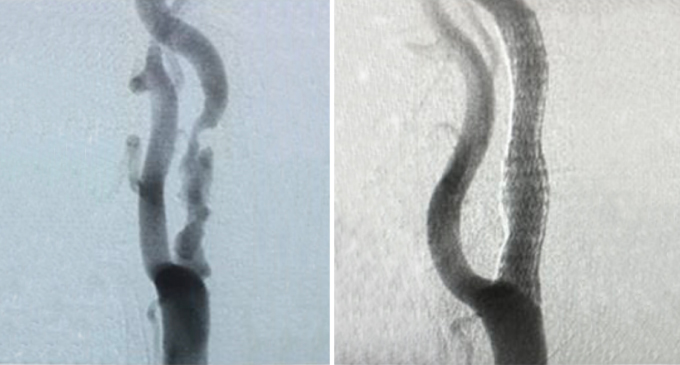
Superb Lesion Coverage
Open cell design and autoconforming technology provide durable plaque coverage.
Instructions for use
The ENROUTE Transcarotid Stent Stystem used in conjunction with the ENROUTE Transcarotid Neuroprotection System (NPS) is indicated for the treatment of patients at high risk and standard risk for adverse events from carotid endarterectomy.
Discover our other products

ENROUTE® Transcarotid Neuroprotection System
Enables the reversal of blood during the TCAR procedure to protect the brain from potential emboli.

ENROUTE Enflate® Transcarotid RX Balloon Dilatation Catheter
The only transcarotid RX balloon catheter available in the US market, designed to improve the efficiency and predictability of the TCAR procedure.
ENROUTE® 0.014” Guidewire
Provides precise lesion navigation in tortuous short vessel segments while performing TCAR.
ENHANCE® Transcarotid Peripheral Access Kit
The only micropuncture access kit developed for the TCAR procedure making it easier and more efficient.
PRESCRIBING INFORMATION
Indications for Use: The ENROUTE® Transcarotid Stent System used in conjunction with the ENROUTE Transcarotid Neuroprotection System (NPS) is indicated for the treatment of patients at high risk for adverse events from carotid endarterectomy, who require carotid revascularization and meet the criteria outlined below.
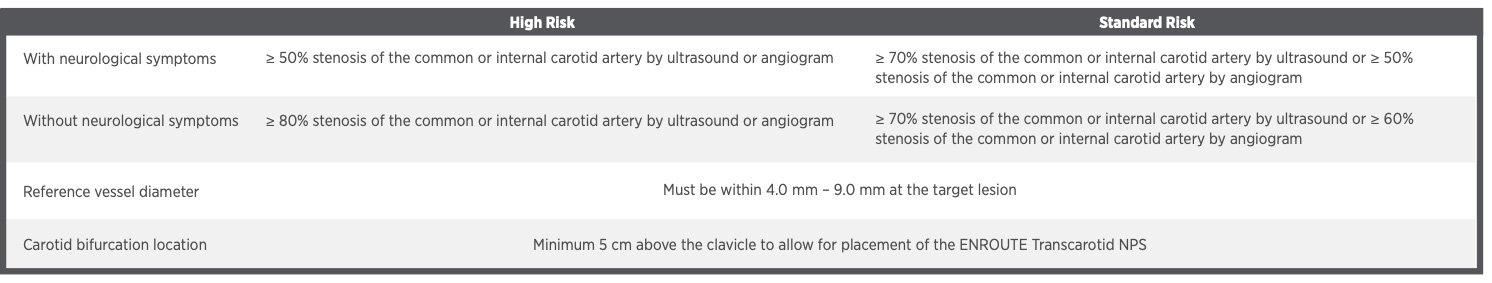
Please refer to Instructions for Use for indications, contraindications, warnings and precautions.
Caution: Federal (U.S.) law restricts this device to sale by or on the order of a physician.
Contraindications: Use of the ENROUTE Transcarotid Stent System is contraindicated in the following patients:
- Patients in whom antiplatelet and/or anticoagulation therapy is contraindicated.
- Patients in whom the ENROUTE Transcarotid NPS is unable to be placed.
- Patients with uncorrected bleeding disorders.
- Patients with known allergies to nitinol.
- Lesions in the ostium of the common carotid artery.
General Warnings
- Only physicians who have received appropriate training for transcarotid stenting and who are familiar with the principles, clinical applications, complications, side effects and hazards commonly associated with carotid interventional procedures should use this device.
- The safety and efficacy of the ENROUTE Transcarotid Stent System have not been demonstrated with embolic protection systems other than the ENROUTE Transcarotid NPS. Use the ENROUTE Transcarotid Stent System only with the ENROUTE Transcarotid NPS.
- The long term performance (> 3 years) of carotid stents has not yet been established.
- As with any type of vascular implant, infection secondary to contamination of the stent may lead to thrombosis, pseudoaneurysm or rupture.
- The stent may cause a thrombus, distal embolization or may migrate from the site of implant through the arterial lumen. Appropriate sizing of the stent to the vessel is required to reduce the
possibility of stent migration (see Section 9.3 of the full instructions for use). In the event of thrombosis of the expanded stent, thrombolysis and PTA should be attempted. - Overstretching of the artery may result in rupture and life-threatening bleeding.
- In patients requiring the use of antacids and/or H2-antagonists before or immediately after stent placement, oral absorption of antiplatelet agents (e.g., aspirin) may be adversely affected.
- The appropriate antiplatelet and anticoagulation therapy should be administered pre- and post-procedure as suggested in Section 9.1 of the full instructions for use.
- In the event of complications such as infection, pseudoaneurysm or fistulization, surgical removal of the stent may be required.
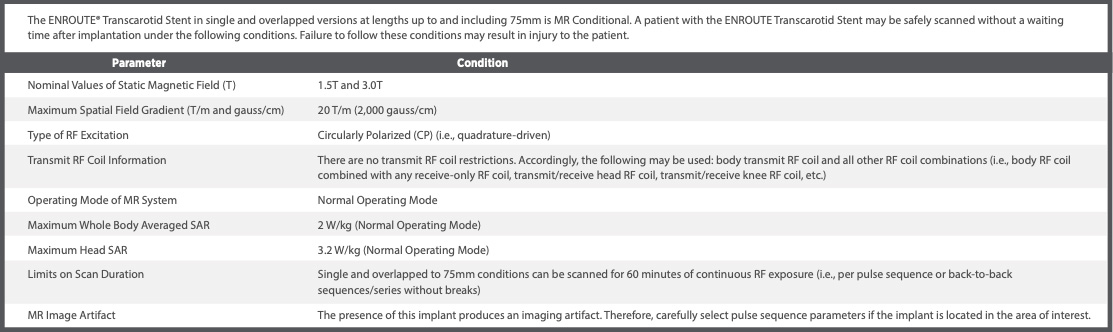
Non-clinical testing has demonstrated that the ENROUTE Transcarotid Stent is MR Conditional.
Adverse Events (in alphabetical order) that may be associated with the use of the ENROUTE Transcarotid Stent System when used in conjunction with the ENROUTE Transcarotid NPS include, but may not be limited to (based upon clinical trial data for the PRECISE Stent System and the ANGIOGUARD XP Emboli Capture Guidewire and clinical trial data from the ROADSTER and PROOF studies):
Air embolism, Allergic/anaphylactoid reaction, Anemia, Aneurysm, Angina/coronary ischemia, Arrhythmia (including bradycardia, possibly requiring need for a temporary or permanent pacemaker), Arterial dissection, Arterial occlusion/restenosis of the treated vessel, Arterial occlusion/thrombus, at puncture site, Arterial occlusion/thrombus, remote from puncture site, Arteriovenous fistula, Atelectasis, Atrial Fibrillation, Bacteremia or septicemia, Cerebral edema, Congestive heart failure, Death, Embolization, arterial, Embolization, stent, Emergent repeat hospital intervention, Fever, Gastrointestinal disorders, GI bleeding from anticoagulation/antiplatelet medication, Hallucination, Hematoma bleed, access site, Hematoma bleed, remote site, Hemorrhage, Hyperperfusion syndrome, Hypotension/hypertension, Hypomagnesaemia, Hypophosphatemia, Infection, Intimal injury/dissection, lschemia/infarction of tissue/organ, Local infection and pain at insertion site, Malposition (failure to deliver the stent to the intended site), Myocardial infarction, Nausea, Oxygen saturation decrease, Pain, Pseudoaneurysm, Rales, Renal failure, Respiratory Infection, Restenosis of the vessel (> 50% obstruction), Rhinorrhea, Seizure, Severe unilateral headache, Stent migration, Stent thrombosis, Stroke, Transient ischemic attack, Transient intolerance to reverse flow, Urinary tract infection, Vasospasm, Venous occlusion/thrombosis, at puncture site, Venous occlusion/thrombosis, remote from puncture site, Vessel rupture, dissection, perforation, Vomiting, Wheezin
Caution: Federal (U.S.) Law restricts this device to sale by or on the order of a physician. ENROUTE and the Silk Road Logo are registered trademarks of Silk Road Medical, Inc. CORDIS®, PRECISE® and ANGIOGUARD® are registered trademarks of Cordis Corporation.

